Retatrutide
RETATRUTIDE | TRIPLE G | GLP1-GIP & GLUCAGON PEPTIDE
Advanced Peptide Therapy for Weight Management and Metabolic Health
Presented by JN Beauty Solutions™
OVERVIEW
Retatrutide is an investigational peptide developed by Eli Lilly for the treatment of obesity, type 2 diabetes, and other metabolic disorders. Unlike conventional weight loss medications, Retatrutide is designed to address the root causes of metabolic imbalance by acting on multiple hormonal pathways simultaneously.
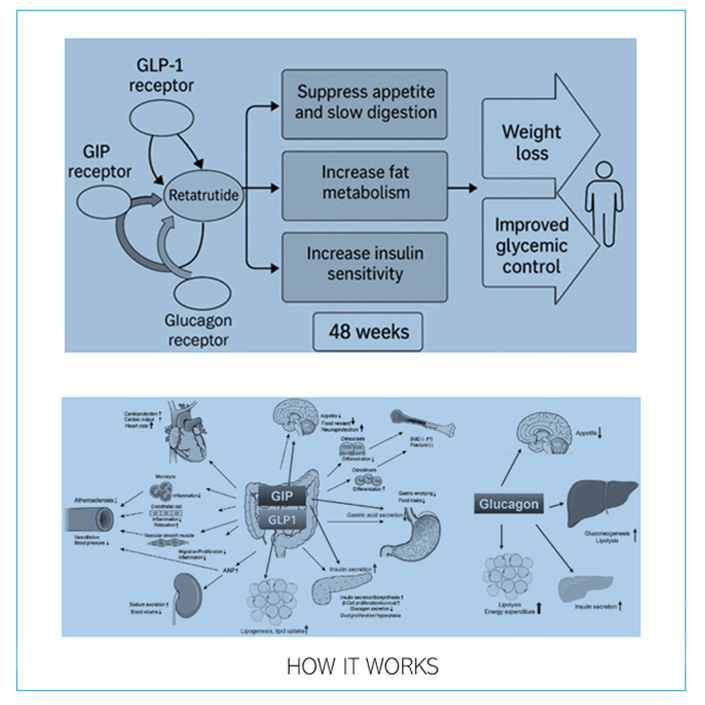
This next-generation therapy is classified as a triple-receptor agonist, meaning it targets GLP-1 (Glucagon-Like Peptide-1), GIP (Glucose-Dependent Insulinotropic Polypeptide), and Glucagon receptors. This unique mechanism provides a comprehensive metabolic reset—enhancing fat metabolism, improving insulin sensitivity, and supporting sustainable weight loss.
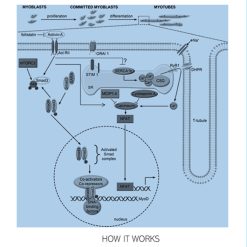
HOW RETATRUTIDE WORKS
After subcutaneous injections, Retatrutide activates three key receptors:
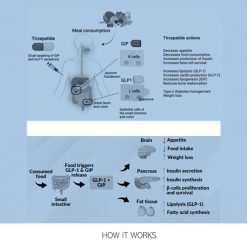
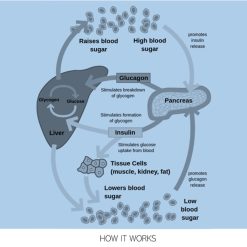
- GLP-1: Delays gastric emptying, reduces appetite, stimulates insulin secretion, and lowers glucagon production.
- GIP: Amplifies insulin release in response to elevated glucose, improves insulin sensitivity, and modulates fat storage.
- Glucagon: Promotes lipolysis, increases energy expenditure through thermogenesis, and enhances fat burning even at rest.
Together, these actions result in reduced calorie intake, improved glycemic control, and greater energy utilization, making Retatrutide one of the most promising agents in modern obesity care.
CLINICAL BENEFITS (According to Preliminary Studies)
- Significant Weight Reduction: Clinical trials report an average weight loss of up to 24% after 48 weeks of treatment, exceeding results seen with Semaglutide or Tirzepatide.
- Improved Blood Sugar Control: Retatrutide has demonstrated a marked reduction in HbA1c levels and insulin resistance in patients with type 2 diabetes.
- Cardiometabolic Improvements: The compound helps lower visceral fat, improve lipid profiles, and reduce blood pressure.
- Enhanced Fat Metabolism: By stimulating glucagon receptors, Retatrutide increases fat oxidation and helps preserve lean muscle mass during weight loss.
- Potential for NAFLD/NASH Management: Preliminary data also suggest benefits in reducing liver fat and improving liver function markers.
ADMINISTRATION
- Dosage Form: Subcutaneous injection (weekly)
- Typical Starting Dose: 2 mg per week, with gradual titration up to 12 mg/week, depending on tolerance and clinical goals.
COMMON SIDE EFFECTS
- Gastrointestinal disturbances such as nausea, vomiting, and diarrhea, particularly during dose escalation.
- Dizziness or fatigue, related to reduced blood pressure.
- Potential muscle loss if protein intake and resistance training are not properly maintained.
WHO SHOULD NOT USE RETATRUTIDE?
Retatrutide is contraindicated in the following populations:
- Individuals with a personal or family history of medullary thyroid carcinoma (MTC) or multiple endocrine neoplasia type 2 (MEN2).
- Patients with a history of pancreatitis.
- Those with severe hepatic or renal impairment.
- Pregnant or breastfeeding women, due to insufficient safety data.
- Individuals with known hypersensitivity to GLP-1, GIP, or glucagon analogs.
- Patients with severe gastrointestinal disorders or a history of severe hypoglycemia.
HOW DOES RETATRUTIDE COMPARE TO OTHER PEPTIDES?
While Semaglutide (GLP-1 agonist) and Tirzepatide (GLP-1 + GIP dual agonist) have set new standards in obesity management, Retatrutide may surpass them by incorporating glucagon receptor activation as well. This triple-action mechanism not only boosts weight loss efficacy but also enhances metabolic flexibility and energy expenditure.
In early trials, Retatrutide consistently demonstrated greater reductions in body weight and blood sugar markers compared to its predecessors. However, it is still in Phase 3 clinical development and not yet approved for public use.
CONCLUSION
Retatrutide represents a breakthrough in metabolic medicine—offering a holistic, multi-targeted approach to treating obesity and type 2 diabetes. With its ability to suppress appetite, regulate glucose, and stimulate fat metabolism, Retatrutide has the potential to become the most powerful weight loss peptide to date.
Pending FDA approval, Retatrutide is positioned to redefine standards in the management of chronic metabolic diseases.
For more information, please contact
JN Beauty Solutions™
Be the first to review “Retatrutide” Cancel reply
Related products
Peptide Generics
Peptide Generics
Peptide Generics
Peptide Generics
Peptide Generics
Peptide Generics



Reviews
There are no reviews yet.