OVERVIEW
Tirzepatide is a novel dual-incretin peptide developed by Eli Lilly, designed to treat type 2 diabetes and obesity. It combines the activity of two key gut hormones—glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1)—into a single molecule, offering a synergistic approach to improving glycemic control and inducing substantial weight loss.
Approved under the brand names Mounjaro® (for type 2 diabetes) and recently Zepbound™ (for chronic weight management), Tirzepatide is currently regarded as one of the most effective medications in the incretin-based therapy class.
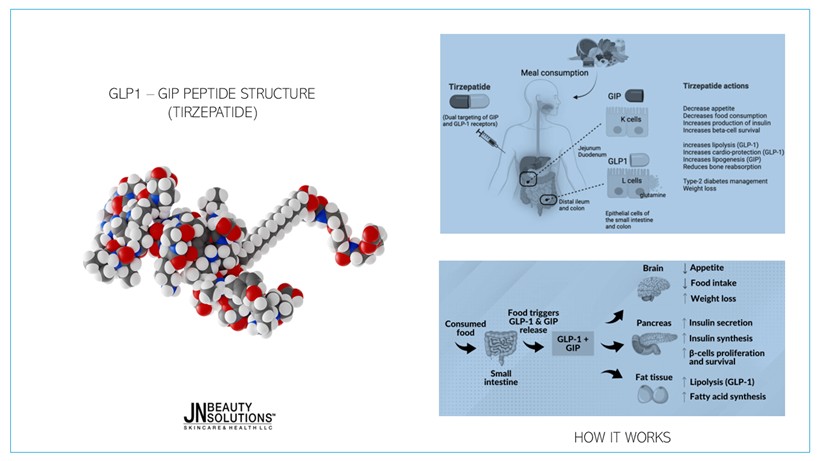
MECHANISM OF ACTION
Tirzepatide functions as a dual agonist that binds to both GIP and GLP-1 receptors:
- GIP Receptor Activation
- Enhances glucose-stimulated insulin secretion
- Improves insulin sensitivity in peripheral tissues
- Modulates lipid metabolism and reduces fat accumulation
- GLP-1 Receptor Activation
- Stimulates insulin secretion
- Inhibits glucagon release during hyperglycemia
- Slows gastric emptying and suppresses appetite via central nervous system action
Together, these mechanisms promote efficient blood sugar regulation, appetite reduction, and body weight loss, while also improving cardiometabolic health.
PHARMACOLOGICAL DESIGN & PHARMACOKINETICS
Tirzepatide is a synthetic linear peptide that includes a modified GIP backbone with GLP-1 activity. It is conjugated to a C20 fatty diacid moiety, extending its half-life and enabling once-weekly subcutaneous dosing.
Half-life: Approximately 5 days
Peak plasma concentration: Achieved in 24–48 hours post-injection
Steady-state levels: Reached after 4–5 weeks of continuous administration
This allows for consistent weekly administration with stable therapeutic levels.
CLINICAL RESEARCH HIGHLIGHTS
1. SURPASS Program – Type 2 Diabetes Trials
- Across multiple SURPASS trials, Tirzepatide demonstrated:
- Superior HbA1c reductions compared to GLP-1 monotherapy (Semaglutide)
- Mean HbA1c reductions of up to 2.4% at the highest dose (15 mg/week)
- High rates of achieving HbA1c < 7.0% and < 6.5% without significant hypoglycemia
- Notably, in SURPASS-2, Tirzepatide outperformed Semaglutide (1.0 mg) in both glycemic control and weight loss, marking a significant advancement in the class.
2. SURMOUNT Program – Obesity Trials
In non-diabetic individuals with obesity (BMI ≥30), the SURMOUNT-1 trial showed:
- Mean body weight reduction of up to 22.5% at 15 mg/week after 72 weeks
- Over 85% of participants achieved ≥5% weight loss
- Clinically meaningful improvements in waist circumference, lipids, and blood pressure
- These outcomes position Tirzepatide as a leading anti-obesity therapy alongside lifestyle interventions.
METABOLIC AND CARDIOVASCULAR BENEFITS
In addition to weight and glycemic benefits, Tirzepatide improves several metabolic and cardiovascular markers:
- Reduction in triglycerides, LDL cholesterol, and blood pressure
- Improved HOMA-IR and insulin resistance indices
- Favorable impact on inflammatory markers associated with metabolic syndrome
- Further long-term data are being investigated in ongoing cardiovascular outcome trials (CVOTs), including SURPASS-CVOT.
ADMINISTRATION & DOSAGE
- Form: Subcutaneous injection, once weekly
- Starting Dose: 2.5 mg/week
- Maintenance Doses: 5 mg, 10 mg, or 15 mg/week (based on titration and tolerability)
- Dose escalation typically occurs in 2.5 mg increments every 4 weeks.
SAFETY AND TOLERABILITY
Common adverse effects:
- Gastrointestinal symptoms: Nausea, vomiting, diarrhea (especially during early dose escalation)
- Mild hypoglycemia: Primarily in combination with sulfonylureas or insulin
- Injection-site reactions, fatigue, decreased appetite
Contraindications:
- Personal or family history of medullary thyroid carcinoma (MTC) or MEN2
- History of pancreatitis
- Severe gastrointestinal disease or gastroparesis
- Not recommended during pregnancy or breastfeeding
COMPARISON WITH SEMAGLUTIDE
While both Tirzepatide and Semaglutide are incretin-based therapies, Tirzepatide’s dual-receptor mechanism provides superior clinical outcomes in many studies:
- Greater HbA1c reductions
- Higher percentage of patients achieving target weight loss
- Enhanced insulin sensitivity and lipid control
This makes Tirzepatide a compelling option for patients requiring both glucose and weight management support.
CONCLUSION
Tirzepatide represents a paradigm shift in the treatment of type 2 diabetes and obesity. With its dual-incretin action, it delivers unmatched efficacy in lowering blood sugar and body weight, with additional metabolic and cardiovascular benefits.
As clinical data continues to evolve, Tirzepatide is expected to become a foundational therapy for chronic metabolic disease, supporting long-term health outcomes across diverse patient populations.
For more information, please contact
JN Beauty Solutions™

